
Acumed
About Acumed
Acumed is a company that focuses on the design and manufacture of innovative orthopaedic fixation solutions in the healthcare industry. The company's main offerings include a wide range of products for different parts of the body such as the foot, ankle, hand, wrist, elbow, and shoulder, which are used to improve patient care by treating various types of fractures and injuries. It is based in Hillsboro, Oregon.
Loading...
Loading...
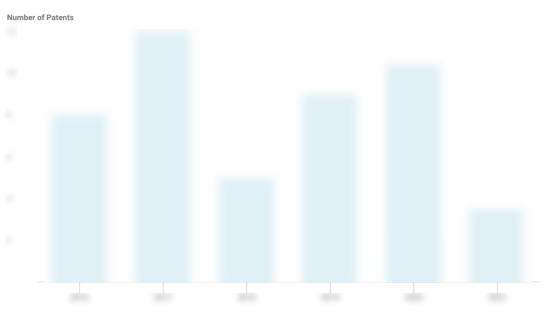
Acumed Patents
Acumed has filed 134 patents.
The 3 most popular patent topics include:
- orthopedic surgical procedures
- bone fractures
- skeletal system
Application Date | Grant Date | Title | Related Topics | Status |
|---|---|---|---|---|
2/4/2022 | 9/3/2024 | Bone fractures, Automotive transmission technologies, Mechanics, Thread standards, Force | Grant |
Application Date | 2/4/2022 |
|---|---|
Grant Date | 9/3/2024 |
Title | |
Related Topics | Bone fractures, Automotive transmission technologies, Mechanics, Thread standards, Force |
Status | Grant |
Latest Acumed News
Aug 27, 2024
| DelveInsight News provided by Share this article Share toX The growth in the heart closure devices market from 2024-2030 is mainly driven by several factors: the rising incidence of congenital heart defects, increased use of MRI procedures, greater awareness of cardiac irregularities in children, and ongoing innovations in product development. These elements collectively contribute to the heart closure devices market's expansion during the forecast period. LAS VEGAS, Aug. 27, 2024 /PRNewswire/ -- DelveInsight's Heart Closure Devices Market Insights report provides the current and forecast market analysis, individual leading heart closure devices companies' market shares, challenges, heart closure devices market drivers, barriers, trends, and key market heart closure devices companies in the market. Key Takeaways from the Heart Closure Devices Market Report As per DelveInsight estimates, North America is anticipated to dominate the global heart closure devices market during the forecast period. In the closure type segment of the heart closure devices market, the patent foramen ovale category (PFO closure) had a significant revenue share in the year 2023. Notable heart closure devices companies such as Abbott, W. L. Gore & Associates, Inc., Boston Scientific Corporation, Occlutech, Heartstitch, SMT, Cardia, Inc., Lifetech Scientific, Lepu Medical Technology(Beijing)Co., Ltd., ATRICURE, INC., and several others, are currently operating in the heart closure devices market. In September 2022, Abbott announced the European launch of its Amplatzer™ Talisman™ PFO Occlusion System to treat people with a patent foramen ovale (PFO), a hole in the heart that doesn't close following birth who have experienced a stroke and are at risk of having another. In September 2021, Abbott announced that the FDA has approved its patent foramen ovale occlusion system for patients with a PFO and prior stroke at risk for recurrent ischemic stroke. To read more about the latest highlights related to the heart closure devices market, get a snapshot of the key highlights entailed in the Global Heart Closure Devices Market Report Heart Closure Devices Overview Heart closure devices are medical tools designed to treat structural defects in the heart, such as atrial septal defects (ASD), patent foramen ovale (PFO), and ventricular septal defects (VSD). These devices are typically small, mesh-like structures made of metal and synthetic materials. They are deployed via a minimally invasive procedure known as cardiac catheterization, where the device is guided through blood vessels to the heart using a catheter. Once in place, the device expands to cover and seal the defect, promoting tissue growth over time that fully closes the opening. This method reduces the need for open-heart surgery, decreasing recovery times and associated risks. The development and utilization of heart closure devices have significantly improved the prognosis for patients with congenital heart defects. These devices not only mitigate symptoms such as fatigue, breathlessness, and stroke risk but also enhance overall heart function. Innovations in this field continue to evolve, with newer devices becoming more effective and easier to implant. Clinical trials and long-term studies have demonstrated the safety and efficacy of heart closure devices, making them a standard treatment option. As technology advances, these devices will likely become even more refined, offering hope and a better quality of life for patients with heart defects. Heart Closure Devices Market Insights In 2023, North America held the largest share of the heart closure devices market. This is attributed to several key factors: a rise in congenital heart defects among children, increasing awareness programs and initiatives for children with cardiac abnormalities, high disposable income, advanced healthcare infrastructure, the presence of key market players, and heightened product development activities. These elements are expected to drive market growth in the region from 2024 to 2030. According to the Centers for Disease Control and Prevention (CDC) in 2023, congenital heart defects (CHDs) affect nearly 1% of births in the United States, equating to about 40,000 births per year. Every 15 minutes, a baby is born with a congenital heart defect in the U.S. The CDC also reports that over 2.4 million children and adults in the U.S. currently live with congenital heart defects. Annually, approximately 5,240 babies in the U.S. are born with an atrial septal defect, representing about 1 in every 769 births. Additionally, around 16,800 babies are born each year with a ventricular septal defect, or 1 in every 240 births. The increase in product approvals and launches further supports market growth. For example, in September 2023, the FDA approved the WATCHMAN FLX™ Pro Left Atrial Appendage Closure Device by Boston Scientific Corporation. These factors are anticipated to drive the growth of the heart closure devices market in North America during the forecast period from 2024 to 2030. To know more about why North America is leading the market growth in the heart closure devices market, get a snapshot of the Heart Closure Devices Market Outlook The heart closure devices market has experienced significant growth in recent years, driven by advancements in medical technology and an increasing prevalence of cardiovascular diseases. These devices, which include products like septal occluders, left atrial appendage closure devices, and patent foramen ovale (PFO) closure devices, are essential in the minimally invasive treatment of congenital heart defects and in the prevention of stroke and other complications associated with abnormal openings in the heart. The rising incidence of heart-related conditions, particularly in aging populations, has propelled the demand for effective and less invasive therapeutic options, thereby boosting the market for heart closure devices. Technological innovations have been pivotal in shaping the heart closure devices market. The development of next-generation devices with enhanced precision, safety, and ease of use has broadened the scope of procedures that can be performed percutaneously. For instance, advanced imaging techniques and better delivery systems have improved the accuracy of device placement, minimizing risks and improving patient outcomes. Additionally, the integration of biocompatible materials has reduced the incidence of complications such as device-related thrombosis or infection, further driving the adoption of these devices in clinical practice. Regulatory approvals and the expanding indications for the use of heart closure devices have also contributed to market growth. The FDA and other regulatory bodies worldwide have approved several devices for broader applications, including stroke prevention in patients with atrial fibrillation who are contraindicated for anticoagulant therapy. These approvals not only validate the safety and efficacy of the devices but also open up new patient segments, thereby expanding the market. Furthermore, favorable reimbursement policies in many countries are making these procedures more accessible, encouraging both patients and healthcare providers to opt for these advanced treatments. Market dynamics are also influenced by the competitive landscape, characterized by the presence of several key players and continuous mergers and acquisitions. Major companies like Abbott Laboratories, Boston Scientific Corporation, and W.L. Gore & Associates are at the forefront, investing heavily in research and development to innovate and expand their product portfolios. Collaborations and strategic partnerships are common, aiming to leverage each other's strengths and accelerate the introduction of new products. This competitive environment fosters innovation and drives down costs, making heart closure devices more affordable and widely available. However, the market does face challenges, including high costs of devices and procedures, stringent regulatory requirements, and the need for specialized training for healthcare professionals. Despite these hurdles, the overall outlook for the heart closure devices market remains positive. Continuous technological advancements, increasing awareness about minimally invasive procedures, and growing healthcare expenditure are expected to sustain the market's growth trajectory. As the global burden of cardiovascular diseases continues to rise, the demand for effective heart closure solutions will likely remain strong, driving further innovations and market expansion. USD 3.99 Billion Key Heart Closure Devices Companies Abbott, W. L. Gore & Associates, Inc., Boston Scientific Corporation, Occlutech, Heartstitch, SMT, Cardia, Inc., Lifetech Scientific, Lepu Medical Technology(Beijing)Co., Ltd., ATRICURE, INC., among others Heart Closure Devices Market Assessment Heart Closure Devices Market Segmentation Heart Closure Devices Market Segmentation By Closure Type: Congenital Heart Defect Closure [Atrial Septal Defect (ASD), Patent Ductus Arteriosus (PDA), and Ventricular Septal Defect (VSD), Patent Foramen Ovale (PFO Closure), and Left Atrial Appendage (LAA) Closure Heart Closure Devices Market Segmentation By Geography: North America, Europe, Asia-Pacific, and Rest of World Porter's Five Forces Analysis, Product Profiles, Case Studies, KOL's Views, Analyst's View Which MedTech key players in the heart closure devices market are set to emerge as the trendsetter explore @ Heart Closure Devices Companies Table of Contents Disclaimer & Contact Us Interested in knowing the heart closure devices market by 2030? Click to get a snapshot of the Heart Closure Devices Market Trends Related Reports Patent Foramen Ovale (PFO) Closure Devices Market Insight, Competitive Landscape, and Market Forecast – 2030 report delivers an in-depth understanding of market trends, market drivers, market barriers, and key PFO closure devices companies, including Abbott, W. L. Gore & Associates, Inc., Occlutech Holding AG, SMT, and Cardia Inc., Lepu Medical Technology(Beijing)Co.,Ltd., Lifetech Scientific, AGA Medical Corporation, pfm medical ag, and Starway Medical Technology Inc., among others. Left Atrial Appendage Closure Device Market Insight, Competitive Landscape, and Market Forecast – 2030 report delivers an in-depth understanding of market trends, market drivers, market barriers, and key left atrial appendage closure devices companies, including Boston Scientific Corporation, Johnson & Johnson, Abbott Laboratories, Articure, Occlutech, Cardia Inc., Lifetech Scientific Corporation, Lepu Medical Technology, Biosense Webster, SentreHEART, Inc., Conformal Medical, and Lifetech Scientific Corporation, among others. Arteriotomy Closure Devices Market Insight, Competitive Landscape, and Market Forecast – 2030 report delivers an in-depth understanding of market trends, market drivers, market barriers, and key arteriotomy closure devices companies, including Abbott Vascular, Cardiva Medical, Arstatis, Cardinal Health, St. Jude Medical, Morris Innovative Research, Scion BioMedical, among others. Sternal Closure Device Market Insight, Competitive Landscape, and Market Forecast – 2030 report delivers an in-depth understanding of market trends, market drivers, market barriers, and key sternal closure devices companies, including Zimmer Biomet, KLS Martin Group., Stryker, Acumed LLC, Crown Holdco One, Inc., B. Braun SE, JEIL MEDICAL CORPORATION., Johnson & Johnson Services, Inc., IDEAR S.R.L, Medtronic, ABYRX, INC., MediGroup EB, A&E Medical Corporation, Medline Industries, LP., Medicon eG., Arthrex, Inc., Able Medical Devices Inc., CircumFix Solutions, Inc., Kinamed® Incorporated., Changzhou Waston Medical Appliance Co, Ltd., among others. About DelveInsight DelveInsight is a leading Business Consultant, and Market Research firm focused exclusively on life sciences. It supports pharma companies by providing comprehensive end-to-end solutions to improve their performance. Contact Us
Acumed Frequently Asked Questions (FAQ)
Where is Acumed's headquarters?
Acumed's headquarters is located at 5885 Nw Cornelius Pass Rd., Hillsboro.
Who are Acumed's competitors?
Competitors of Acumed include DJO and 1 more.
Loading...
Compare Acumed to Competitors

FLSmidth is a global provider of technology and services for the mining and cement industries, focusing on performance improvement and environmental impact reduction. The company offers a full flowsheet of equipment and solutions designed to enhance productivity and support zero-emissions mining and cement production. FLSmidth serves a diverse range of sectors within the mining and cement industries, providing solutions to improve product quality, increase capacity, and minimize environmental footprint. FLSmidth was formerly known as FFE Minerals. It was founded in 1882 and is based in Copenhagen, Denmark.
Arthrex is a global medical device company specializing in the development of products and educational programs for orthopedic surgery. The company offers a range of innovative products designed to improve minimally invasive orthopedic procedures and provides medical education to advance surgical techniques. Arthrex primarily serves the healthcare sector, focusing on quality and innovation in orthopedics. It was founded in 1981 and is based in Naples, Florida.
Otto Bock HealthCare focuses on medical technology, specifically in the prosthetics, orthotics, and mobility sectors. The company offers a range of products including arm and leg prosthetics, mobility aids such as wheelchairs, and orthotic devices like braces and supports, all designed to enhance the mobility and independence of individuals with physical disabilities. It was founded in 1919 and is based in Duderstadt, Germany.
Medline operates as a healthcare company. It specializes in manufacturing and distributing medical products and solutions. They provide a range of quality medical products that are essential for patient care in various healthcare settings. Medline primarily serves the healthcare industry, offering products that help improve clinical and supply chain operations. It was founded in 1966 and is based in Northfield, Illinois.
Orthofix is a medical device company that operates in the spine and orthopedics sector. The company provides reconstructive and regenerative solutions for various spinal and bone-related conditions, and offers minimally invasive extremity solutions to improve patient's quality of life. These solutions incorporate different treatment modalities such as mechanical, biological, and electromagnetic to achieve desired clinical outcomes. Additionally, Orthofix offers products for pediatrics, limb reconstruction, trauma/fracture management, and foot and ankle specialties. It was founded in 1980 and is based in Lewisville, Texas.

Zimmer Biomet operates as a medical technology company. It designs, develops, manufactures, and markets orthopedic reconstructive products, sports medicine, biologics, extremities, and trauma products, spine, bone healing, craniomaxillofacial, and thoracic products, dental implants, and related surgical products. Zimmer Biomet was formerly known as Zimmer. It was founded in 1927 and is based in Warsaw, Indiana.
Loading...