
Alzheon
Founded Year
2013Stage
Series E | AliveTotal Raised
$241.97MLast Raised
$100M | 2 yrs agoAbout Alzheon
Alzheon focuses on the development of medicines for neurodegenerative disorders, specifically within the healthcare and biotechnology sectors. The company's main product is an oral small-molecule prodrug that aims to block the formation of neurotoxic soluble amyloid oligomers in the brain, which is a key factor in the progression of Alzheimer's disease. Alzheon primarily serves the healthcare industry, with a specific focus on patients suffering from Alzheimer's disease and other neurological disorders. It was founded in 2013 and is based in Framingham, Massachusetts.
Loading...
Loading...
Expert Collections containing Alzheon
Expert Collections are analyst-curated lists that highlight the companies you need to know in the most important technology spaces.
Alzheon is included in 1 Expert Collection, including Unicorns- Billion Dollar Startups.
Unicorns- Billion Dollar Startups
1,244 items
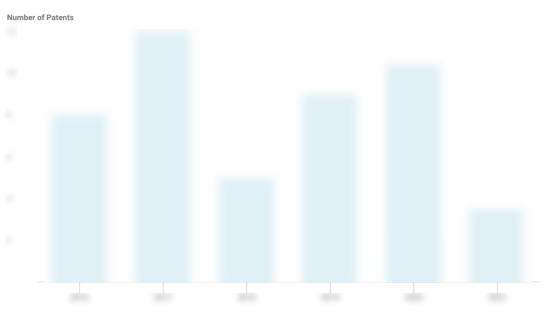
Alzheon Patents
Alzheon has filed 14 patents.
The 3 most popular patent topics include:
- rare diseases
- neurological disorders
- alzheimer's disease
Application Date | Grant Date | Title | Related Topics | Status |
|---|---|---|---|---|
7/30/2019 | 9/10/2024 | Alzheimer's disease, Rare diseases, Neurodegenerative disorders, Cognitive disorders, Psychiatric diagnosis | Grant |
Application Date | 7/30/2019 |
|---|---|
Grant Date | 9/10/2024 |
Title | |
Related Topics | Alzheimer's disease, Rare diseases, Neurodegenerative disorders, Cognitive disorders, Psychiatric diagnosis |
Status | Grant |
Latest Alzheon News
Sep 6, 2024
September 6, 2024 Article ALZ-801 has now progressed to the phase 3 APOLLOE4 study, designed to evaluate efficacy, safety, and biomarker and imaging effects. In addition to the growing body of infusion-based treatments for Alzheimer disease, a new, oral option has shown encouraging results in clinical trials. Valiltramiprosate (ALZ-801; Alzheon) is an investigational disease-modifying therapy currently in phase 3 development. When administered at the phase 3 clinical dose, ALZ-801 was able to fully block the formation of neurotoxic soluble beta amyloid (Ab) oligomers and has shown potential for patients with 2 copies of the apolipoprotein e4 allele (APOE4). This patient population is the focus of the 78-week APOLLOE4 phase 3 trial.1 A woman holding a pill | Image credit: fizkes | stock.adobe.com “What’s striking is that all of these proteins that ultimately become toxic have a very important function in brain health, for normal brain function,” Martin Tolar, MD, PhD, founder, president, and CEO or Alzheon, said in an interview. “The monomeric amyloid, which is about 1% of our brains, is a system that protects the brain against injury, infection, stress, you name it. The problem…is if you lose the ability in your 40s and 50s to [effectively clear] these proteins from the brain.” Importantly, 15% of patients with Alzheimer disease are APOE4/4 homozygous and two-thirds of patients are APOE4 carriers. This patient population can experience clinical onset as much as 10 years earlier than non-carriers, highlighting the significant unmet medical need for this subset of patients.2 In an open-label, multicenter, single-arm phase 2 biomarker trial, researchers found that there was a statistically significant and clinically relevant reduction in plasma biomarkers of neurodegeneration, preservation of brain volume, and encouraging cognitive effects in patients with early Alzheimer disease who are carriers of APOE4, following 24 months of treatment.1 At baseline, trial participants were 52% female with a mean age of 69 years, a mean Mini-Mental Status Examination (MMSE) score of 26 (range 22-30 years), and 70% with cognitive impairment. A total of 70 participants completed the week 104 visit and 68 provided evaluable plasma for biomarker assays, allowing inclusion in the study’s primary analysis.2 Participants received the ALZ-801 265 mg tablet once daily for 2 weeks and twice daily thereafter over the 104-week treatment period.1 Notably, investigators saw early, sustained, and statistically significant reductions in plasma P-tau181 reaching 31% at 24 months. They also observed 28% preservation of hippocampal volume compared with external controls of matched subjects, supporting the drug’s neuroprotective effects. Additionally, participants performed better on cognitive tests at 6 months and showed sustained stabilization of cognition above baseline for 24 months.1 In a news release, Tolar said, “[These data] reinforces our conviction that ALZ-801 has the potential to disrupt the Alzheimer treatment paradigm by halting the progression of this relentless and debilitating disease.”1 The study also showed a favorable safety profile with no events of vasogenic edema,1 which is particularly notable given the significant adverse effects seen with other investigational and available treatments for Alzheimer disease. ALZ-801 has now progressed to the phase 3 APOLLOE4 study, designed to evaluate the efficacy, safety, and biomarker and imaging effects of ALZ-801 265 mg twice daily in patients with early Alzheimer disease and 2 copies of APOE4. Topline findings are expected in 2024.3 This 78-week study enrolled patients between the ages of 50 and 80 years with MMSE scores of 22 or greater and Clinical Dementia Rating-Global Scores of 0.5 or 1. In total, 325 participants were enrolled with a mean age of 60 years, 51% female, a mean MMSE of 25.6, and 65% cognitive impairment.3 Although the brain cannot regenerate, Tolar said ALZ-801 is showing exciting potential to prevent the accumulation of damage in patients with Alzheimer disease. “Our goal has always been to have a treatment that can be used preventively for a very long time and in patients way before they have the onset of the clinically terminal stage of the disease,” Tolar said. “In clinical studies, with all of the anti-amyloid treatments that have shown efficacy, you have better efficacy the earlier you start treating. Now, we need to prove to regulatory agencies efficacy at the beginning of this terminal stage.” REFERENCES 1. Alzheon Reports Industry-Leading Biomarker, Brain Preservation and Clinical Benefits Following 24 Months of Treatment in Phase 2 Trial of Oral ALZ-801 (Valiltramiprosate) in Patients With Early Alzheimer’s Disease. News release. Alzheon. September 13, 2023. Accessed September 6, 2024. https://alzheon.com/alzheon-reports-industry-leading-biomarker-brain-preservation-and-clinical-benefits-following-24-months-of-treatment-in-phase-2-trial-of-oral-alz-801-valiltramiprosate-in-patients-with-early-alzhei/ 2. Valiltramiprosate/ALZ-801 potentially the first oral disease-modifying treatment for Alzheimer’s disease. Alzheon. Accessed September 6, 2024. https://alzheon.com/pipeline/alzheon-alz-801/ 3. Abushakra S, Posteinsson AP, Sabbagh M, et al. APOLLOE4 phase 3 study of oral AlZ-801/valiltramiprosate in APOE e4/e4 homozygotes with early Alzheimer’s disease: trial design and baseline characteristics. Alzheimers Dement (NY). 2024;10(3):e12498. doi:10.1002/trc2.12498 Recent Videos
Alzheon Frequently Asked Questions (FAQ)
When was Alzheon founded?
Alzheon was founded in 2013.
Where is Alzheon's headquarters?
Alzheon's headquarters is located at 111 Speen Street, Framingham.
What is Alzheon's latest funding round?
Alzheon's latest funding round is Series E.
How much did Alzheon raise?
Alzheon raised a total of $241.97M.
Who are the investors of Alzheon?
Investors of Alzheon include Alerce Medical Technology Partners, National Institute on Aging, Paycheck Protection Program, Ally Bridge Group and ARCH Venture Partners.
Loading...
Loading...