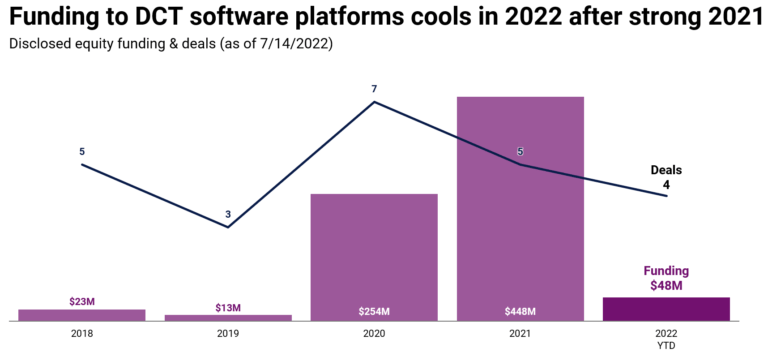
Hawthorne Effect
Founded Year
2015Stage
Series A | AliveTotal Raised
$33.35MLast Raised
$20M | 3 yrs agoMosaic Score The Mosaic Score is an algorithm that measures the overall financial health and market potential of private companies.
+11 points in the past 30 days
About Hawthorne Effect
Hawthorne Effect specializes in providing mobile, tech-driven solutions for clinical trials within the healthcare sector. The company offers a platform that facilitates mobile clinical trial visits, enhances patient access, and ensures high-quality data collection through a network of healthcare professionals. Hawthorne Effect primarily serves the biopharma, medtech, and healthcare industries by improving clinical trial efficiency, data quality, and patient diversity. It was founded in 2015 and is based in Lafayette, California.
Loading...
Hawthorne Effect's Product Videos

ESPs containing Hawthorne Effect
The ESP matrix leverages data and analyst insight to identify and rank leading companies in a given technology landscape.
The trial participant engagement platforms market includes digital tools and resources designed to keep patients engaged throughout the clinical trial process. These platforms provide participants with educational content, instructional videos, reminders, and interactive features to enhance their understanding and involvement in the study. By offering easy access to trial information and support, …
Hawthorne Effect named as Challenger among 12 other companies, including Medidata, Curebase, and ClinOne.
Hawthorne Effect's Products & Differentiators
Hawthorne Cloud
Our HIPAA-compliant, cloud-based platform that unifies all aspects of our decentralized clinical trial approach.
Loading...
Research containing Hawthorne Effect
Get data-driven expert analysis from the CB Insights Intelligence Unit.
CB Insights Intelligence Analysts have mentioned Hawthorne Effect in 3 CB Insights research briefs, most recently on Aug 1, 2023.
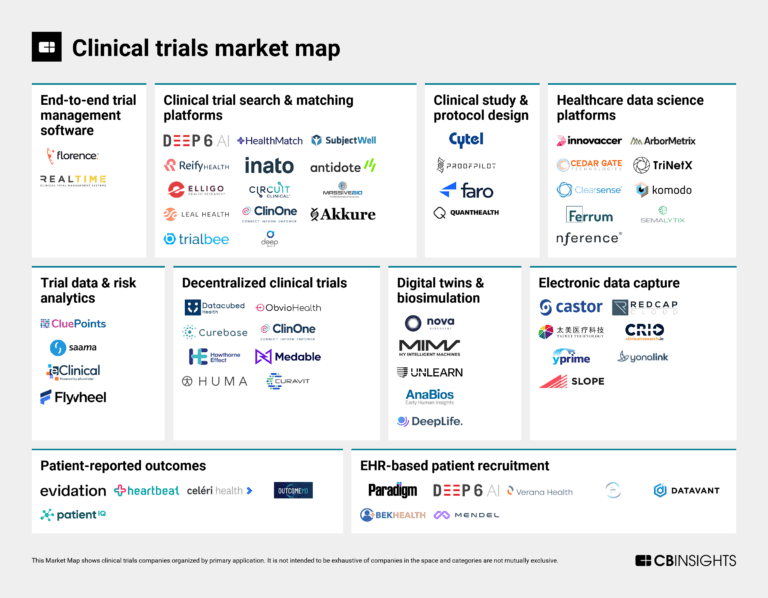
Aug 1, 2023
The clinical trials market mapExpert Collections containing Hawthorne Effect
Expert Collections are analyst-curated lists that highlight the companies you need to know in the most important technology spaces.
Hawthorne Effect is included in 3 Expert Collections, including Conference Exhibitors.
Conference Exhibitors
5,302 items
Digital Health
11,060 items
The digital health collection includes vendors developing software, platforms, sensor & robotic hardware, health data infrastructure, and tech-enabled services in healthcare. The list excludes pureplay pharma/biopharma, sequencing instruments, gene editing, and assistive tech.
Digital Health 50
150 items
The winners of the third annual CB Insights Digital Health 150.
Hawthorne Effect Patents
Hawthorne Effect has filed 1 patent.
The 3 most popular patent topics include:
- clinical research
- clinical trials
- human subject research
Application Date | Grant Date | Title | Related Topics | Status |
|---|---|---|---|---|
10/3/2022 | Clinical research, Medical ethics, Vaccines, Human subject research, Clinical trials | Application |
Application Date | 10/3/2022 |
|---|---|
Grant Date | |
Title | |
Related Topics | Clinical research, Medical ethics, Vaccines, Human subject research, Clinical trials |
Status | Application |
Latest Hawthorne Effect News
Oct 10, 2023
News provided by Share this article Share toX The study marks a new standard for inclusive medicine with recruitment for target demographics reaching over 3x the national average SAN FRANCISCO, Oct. 10, 2023 /PRNewswire/ -- Hawthorne Effect , a complete clinical trials solution, today announced interim recruitment results for the PREVUE-VALVE study , a clinical research study led by the Cardiovascular Research Foundation (CRF), that aims to quantify the prevalence of valvular heart disease (VHD). To date, Hawthorne Effect has successfully recruited over 1,000 patients in 48 states plus Hawaii. Patient recruitment also exceeded industry averages according to findings from the Association of Clinical Research Professionals for the below patient demographics, setting a new standard for inclusiveness: 15% African American compared to the industry standard of <5% 10% Hispanic compared to the industry standard of <1% 3% Asian compared to the industry standard of 2% Despite recent efforts by the FDA to improve diversity in study samples, the clinical trial industry still faces major challenges with diverse patient recruitment and attrition. Black, Latinx, Native American and Asian populations represent 0.5% to <5% of study populations on average, despite representing far larger portions of the United States population. These disparities lead to a research gap in understanding the efficacy of interventions for minority groups in the United States. The true modernization of medicine relies on diverse samples from the entry point: clinical trials. Hawthorne Effect has accomplished these goals by building a platform that prioritizes inclusive patient recruitment and accessible trial execution. "We have overcome decades-long challenges in clinical research with the PREVUE-VALVE study," said Jodi Akin, founder and CEO of Hawthorne Effect. "With a single site of record, we enrolled a 500-patient pilot in less than four months that was nearly representative of the U.S. population and captured data in a short time frame. In doing so, we're making clinical trials more accessible and convenient, and ultimately advancing research to get quality care to all patients faster." The study is supported by the Hawthorne Cloud software platform , an all-in-one digital interface that delivers real-time, validated and highly accurate clinical data from multiple sources. In addition to handling scheduling, equipment logistics and digital enrollment, its intelligent matching algorithm links patients with Hawthorne Heroes , a network of 4,000+ highly-trained medical professionals, who meet the patients where they are, whether it be in their homes, community practices or retail sites. Heroes perform a battery of clinical assessments including in-home echocardiograms (ECG), 12-lead ECG, blood draws and quality of life assessments. This in turn reduces barriers and increases participation for traditionally underrepresented populations. The PREVUE-VALVE study aims to quantify the true prevalence of VHD in a demographic representative sample of the United States population. In addition to Hawthorne Effect and CRF, collaborators include Columbia University, CVS Caremark, University of Michigan, Vanderbilt University Medical Center and retail health partners CVS Health. As populations age worldwide, heart ailments like VHD are increasing, but many patients suffer from delayed or otherwise deficient care despite new and effective therapies. Detection and diagnosis of VHD is critical to successful treatment. The PREVUE-VALVE study was established to correctly determine prevalence and aid healthcare providers in treating VHD at the best possible time. The study aims to be concluded with the publication of the main results by 2025. "This is a major accomplishment for the medical research industry," said Dr. David Cohen, director of clinical and outcomes research at CRF. "For a disease that impacts so many in the U.S., it is critical to gather data that are actually representative of our diverse population. With our partnership with Hawthorne Effect, CRF is paving the way for the development of advanced treatments to keep everyone healthy." Since launching in 2015, Hawthorne Effect has conducted 76 trials of varying complexities, a portion of which were able to be executed during COVID-19 lockdowns by going to patients' households. To learn more about Hawthorne Effect and its modern clinical trials solutions, please visit https://hawthorne-effect.com/ . Trial Design The study is being conducted in a sample of older individuals (i.e., age 65-85 years) that is representative of the U.S. population. The sample will be carefully curated to ensure traditionally underrepresented individuals are included and overrepresented, if possible. For more information on the methodology, visit https://classic.clinicaltrials.gov/ct2/show/NCT05357404 . About Hawthorne Effect Hawthorne Effect is a complete clinical trials solution integrating technology and people to transform the clinical trials industry. Its technology-enabled platform and distributed network of certified medical professionals, Hawthorne Heroes, enable patients and clinicians to participate in trials within their trusted community. Its solution accelerates clinical research and generates evidence that makes clinical trials accessible and convenient while delivering meaningful and high-quality clinical assessments. Since launching in 2015, Hawthorne Effect has helped complete 76 trials and worked with industry leaders within medtech, biopharma, retail health and healthcare systems. Learn more about Hawthorne Effect by visiting https://hawthorne-effect.com/ . Media Contact:
Hawthorne Effect Frequently Asked Questions (FAQ)
When was Hawthorne Effect founded?
Hawthorne Effect was founded in 2015.
Where is Hawthorne Effect's headquarters?
Hawthorne Effect's headquarters is located at 3595 Mt. Diablo Blvd, Lafayette.
What is Hawthorne Effect's latest funding round?
Hawthorne Effect's latest funding round is Series A.
How much did Hawthorne Effect raise?
Hawthorne Effect raised a total of $33.35M.
Who are the investors of Hawthorne Effect?
Investors of Hawthorne Effect include SignalFire, P5 Health Ventures, Northpond Ventures, NuFund Venture Group, Paycheck Protection Program and 3 more.
Who are Hawthorne Effect's competitors?
Competitors of Hawthorne Effect include ObvioHealth, Formation Bio, Clincierge, Science 37, Virtual Monitor and 7 more.
What products does Hawthorne Effect offer?
Hawthorne Effect's products include Hawthorne Cloud and 1 more.
Who are Hawthorne Effect's customers?
Customers of Hawthorne Effect include Hilma.
Loading...
Compare Hawthorne Effect to Competitors

Curebase provides a range of software tools designed for clinical trial recruitment, consent, and data collection processes. Curebase primarily serves the clinical research industry, including CROs, research sites, and study participants. The company was founded in 2017 and is based in San Francisco, California.
Judi is a provider of clinical trial workflow software designed to streamline research processes within the life sciences sector. The company offers a cloud-based platform that facilitates collaboration, data security, and compliance for various stakeholders in clinical trials, including trial sites, sponsors, CROs, and partners. Judi's solutions are tailored to enhance and optimize clinical trial workflows, eliminating manual processes and integrating data across multiple stakeholders. It was founded in 2005 and is based in Boston, Massachusetts.

Reify Health is a company focused on improving the clinical trial process within the healthcare industry. The company offers cloud-based software that accelerates patient enrollment in clinical trials, thereby facilitating the development of new therapies. Reify Health primarily serves the healthcare and biopharma industries. The company was formerly known as ZeroSum Health. It was founded in 2012 and is based in Boston, Massachusetts.

Medable specializes in providing digital clinical trial software solutions within the healthcare and pharmaceutical sectors. The company offers a comprehensive platform that facilitates the management of clinical trials, including tools for remote data collection, electronic consent (eConsent), patient-reported outcomes (ePRO), and clinical outcome assessments (eCOA), all designed to streamline the trial process and enhance data quality. Medable was formerly known as Dermatrap. It was founded in 2012 and is based in Palo Alto, California.

THREAD is a company that focuses on providing a decentralized clinical trial platform in the healthcare and life sciences industry. The company offers a platform and supporting services that enable biopharma, CROs, and life science organizations to remotely capture data from participants and sites during, in-between, and in lieu of in-clinic visits. The platform includes features such as electronic consent (eConsent), electronic clinical outcome assessment (eCOA), sensors, reminders, and telehealth virtual visits. It was founded in 2005 and is based in Tustin, California.

Castor is a medical research data platform. It provides decentralized clinical trial solutions to control clinical trial design. It analyzes, manages, and organizes medical data collected from multiple electronic records. The company was founded in 2012 and is based in New York, New York.
Loading...