
NeoCura
Founded Year
2017Stage
Series A - II | AliveTotal Raised
$116.97MLast Raised
$78.76M | 3 yrs agoAbout NeoCura
NeoCura is committed to building a Ribonucleic acid (RNA) drug platform. It has built a multi-omics big data collection platform and multi-bionics database to perform in-depth drug target mining and fully automated drug design based on Artificial intelligence (AI) algorithms and core bioinformatic technologies. Through application in clinical innovative RNA drug development and next-generation and safe clinical precision diagnosis and treatment, it provides patients with one-stop full-cycle solutions from screening, diagnosis, and treatment to efficacy monitoring. It was founded in 2017 and is based in Beijing, China.
Loading...
NeoCura's Products & Differentiators
NeoCura AG-IND
Neoantigen cancer vaccine for Solid tumor, IND enabling, several investigator initiated clinical trials ongoing in China top tier cancer hospitals
Loading...
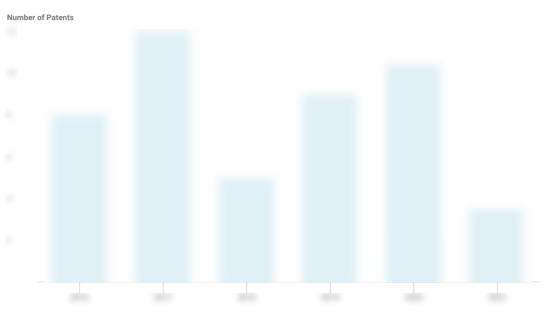
NeoCura Patents
NeoCura has filed 1 patent.
The 3 most popular patent topics include:
- biotechnology
- cell biology
- immunology
Application Date | Grant Date | Title | Related Topics | Status |
|---|---|---|---|---|
8/21/2020 | 3/5/2024 | Immunology, Transcription factors, Cell biology, Biotechnology, Proteins | Grant |
Application Date | 8/21/2020 |
|---|---|
Grant Date | 3/5/2024 |
Title | |
Related Topics | Immunology, Transcription factors, Cell biology, Biotechnology, Proteins |
Status | Grant |
Latest NeoCura News
Apr 19, 2024
Brought to you by XH-001 is under clinical development by NeoCura Bio-Medical Technology and currently in Phase II for Solid Tumor. According to GlobalData, Phase II drugs for Solid Tumor have a 38% phase transition success rate (PTSR) indication benchmark for progressing into Phase III. GlobalData’s report assesses how XH-001’s drug-specific PTSR and Likelihood of Approval (LoA) scores compare to the indication benchmarks. Buy the report here. Smarter leaders trust GlobalData GlobalData tracks drug-specific phase transition and likelihood of approval scores, in addition to indication benchmarks based off 18 years of historical drug development data. Attributes of the drug, company and its clinical trials play a fundamental role in drug-specific PTSR and likelihood of approval. XH-001 overview XH-001 is under development for the treatment advanced solid tumors. It is an mRNA-based tumor neoantigen vaccine and is being developed based on NeoCura Ag, an artificial intelligence (AI) technology. See Also: NeoCura Bio-Medical Technology overview NeoCura Bio-Medical Technology (NeoCura) formerly Shenzhen NeoCura Biotechnology (NeoCura) is an developer of an artificial intelligence (AI) medical platform intended to improve the precision diagnosis and treatment of tumor. NeoCura is headquartered in Shenzhen, Guangdong, China. For a complete picture of XH-001’s drug-specific PTSR and LoA scores, buy the report here. This content was updated on 18 March 2024 Sign up for our daily news round-up! Give your business an edge with our leading industry insights. The gold standard of business intelligence. Blending expert knowledge with cutting-edge technology, GlobalData’s unrivalled proprietary data will enable you to decode what’s happening in your market. You can make better informed decisions and gain a future-proof advantage over your competitors. GlobalData , the leading provider of industry intelligence, provided the underlying data, research, and analysis used to produce this article. GlobalData’s Likelihood of Approval analytics tool dynamically assesses and predicts how likely a drug will move to the next stage in clinical development (PTSR), as well as how likely the drug will be approved (LoA). This is based on a combination of machine learning and a proprietary algorithm to process data points from various databases found on GlobalData’s Pharmaceutical Intelligence Center . Share this article Your corporate email address * Pharma Technology Focus : Pharmaceutical Technology Focus (monthly) Thematic Take (monthly) I consent to Verdict Media Limited collecting my details provided via this form in accordance with Privacy Policy Subscribe Visit our Privacy Policy for more information about our services, how we may use, process and share your personal data, including information of your rights in respect of your personal data and how you can unsubscribe from future marketing communications. Our services are intended for corporate subscribers and you warrant that the email address submitted is your corporate email address. Thank you for subscribing close
NeoCura Frequently Asked Questions (FAQ)
When was NeoCura founded?
NeoCura was founded in 2017.
Where is NeoCura's headquarters?
NeoCura's headquarters is located at Shengmingyuan Road, Life Science Park, Changping District.
What is NeoCura's latest funding round?
NeoCura's latest funding round is Series A - II.
How much did NeoCura raise?
NeoCura raised a total of $116.97M.
Who are the investors of NeoCura?
Investors of NeoCura include Jiayin Fund, Yahui Zhou, CDH Investments, PICC Capital Equity Investment, Shunxi Private Equity Fund Management and 5 more.
Who are NeoCura's competitors?
Competitors of NeoCura include Abogen.
What products does NeoCura offer?
NeoCura's products include NeoCura AG-IND and 4 more.
Loading...
Compare NeoCura to Competitors
Abogen operates as a plant-based pharmaceutical research company. It focuses on the research and development of messenger ribonucleic acid (mRNA) drugs to create antidotes against human disease. The company was founded in 2019 and is based in Suzhou, China.
Loading...