
NeuroLogica
Stage
Acquired | AcquiredTotal Raised
$18MAbout NeuroLogica
NeuroLogica specializes in the design and manufacture of imaging equipment that is easy to use and brings the power of imaging to the patient. NeuroLogica's BodyTom CT and CereTom CT are FDA registered and the quality system is certified to ISO 13485:2003 and ISO 9001:2008 with Canadian Medical Device Amendments. In January 2013, NeuroLogica was acquired by Samsung. The valuation of NeuroLogica was undisclosed. Other terms of the deal were not released.
Loading...
Loading...
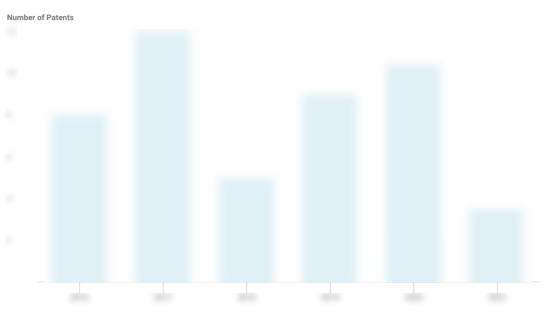
NeuroLogica Patents
NeuroLogica has filed 37 patents.
The 3 most popular patent topics include:
- medical imaging
- medical physics
- imaging
Application Date | Grant Date | Title | Related Topics | Status |
|---|---|---|---|---|
4/12/2022 | 1/30/2024 | Stroke, Medical imaging, Vascular diseases, Cerebrovascular diseases, Medical physics | Grant |
Application Date | 4/12/2022 |
|---|---|
Grant Date | 1/30/2024 |
Title | |
Related Topics | Stroke, Medical imaging, Vascular diseases, Cerebrovascular diseases, Medical physics |
Status | Grant |
Latest NeuroLogica News
Jul 22, 2024
닫기 NeuroLogica Corp., a U.S. subsidiary of Samsung Electronics, received additional U.S. FDA clearance on July 10 for new features of OmniTom Elite Photon Counting Detector (PCD), an advanced CT scanner. The new features include an ultra-high resolution mode, PCD-specific applications, expanded scanning range, and continuous helical scanning capabilities. NeuroLogica Corp., a subsidiary of Samsung Electronics, received additional FDA clearance for the OmniTom Elite Photon Counting Detector (PCD) on July 10, introducing new features such as an ultra-high resolution mode, PCD-specific applications, expanded scanning range, and continuous helical scanning capabilities. The OmniTom Elite's PCD technology is a next-generation CT scanning advancement that uses semiconductors to deliver higher resolution and lower electronic noise in CT images compared to conventional scintillator-based methods. Samsung Medison, a Korean medical device maker uner Samsung Electronics, said that the FDA-cleared upgrade to the OmniTom Elite PCD introduces an ultra-high resolution mode for superior image quality. The imaging can also be adjusted to remove residual contrast seen in CT scans taken after contrast has been administered. The OmniTom Elite PCD was the world's first mobile CT with PCD technology to receive FDA clearance in 2022. “With these new features, the OmniTom Elite PCD is poised to be even more competitive in the global mobile CT market,” Samsung Medison said. “We are pleased to receive additional FDA clearance for the development of new features to improve diagnostic convenience,” said Yoo Kyu-tae, GM of Samsung Electronics HME Business and CEO of Samsung Medison. “We will continue to lead the development of new technologies in the medical imaging field to improve diagnostic accuracy while providing convenience to medical staff. In Korea, the Ministry of Food and Drug Safety approved OmniTom Elite PCD last year, following its existing FDA approval. Meanwhile, the OmniTom Elite PCD is pending to receive CE MDR (European Union Medical Device Regulation) certification for its new FDA-approved features.
NeuroLogica Frequently Asked Questions (FAQ)
Where is NeuroLogica's headquarters?
NeuroLogica's headquarters is located at 14 Electronics Ave, Danvers.
What is NeuroLogica's latest funding round?
NeuroLogica's latest funding round is Acquired.
How much did NeuroLogica raise?
NeuroLogica raised a total of $18M.
Who are the investors of NeuroLogica?
Investors of NeuroLogica include Samsung Electronics and Stata Venture Partners.
Loading...
Loading...